Shanghai Aurefluidics Technology Co.,Ltd. is one of the few domestic companies with independent intellectual property rights in the design of intelligent MEMS microfluidic chips. It is engaged in the design, manufacturing, sales and service of a new generation of intelligent microfluidic products, including printing products and intelligent microfluidic products. It provides overall solutions in the industrial and consumer fields based on its printing chips and control modules with independent intellectual property rights. Based on its world-leading liquid digitization technology, it has developed a variety of intelligent microfluidic chips and control systems, which are widely used in biomedical research, drug R & D and production, clinical medical testing and diagnosis and other fields.

Established in
Office and Lab
Patent Applications
Inventions
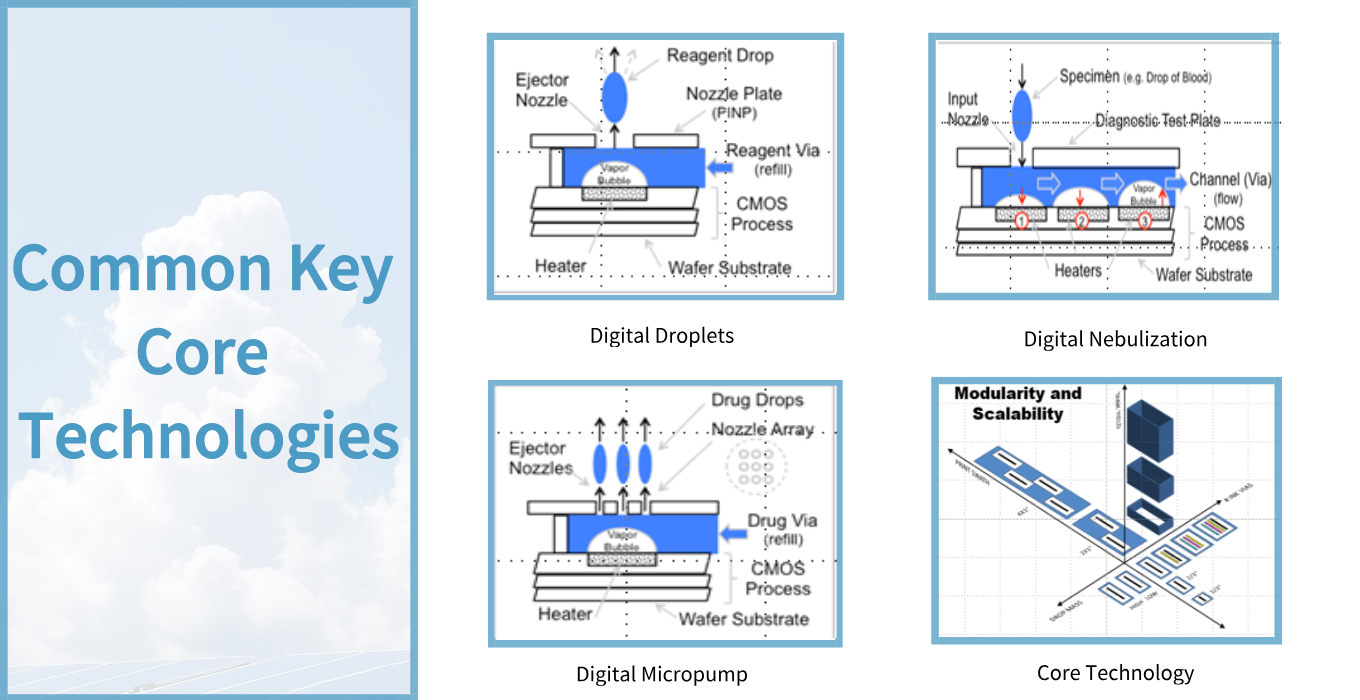
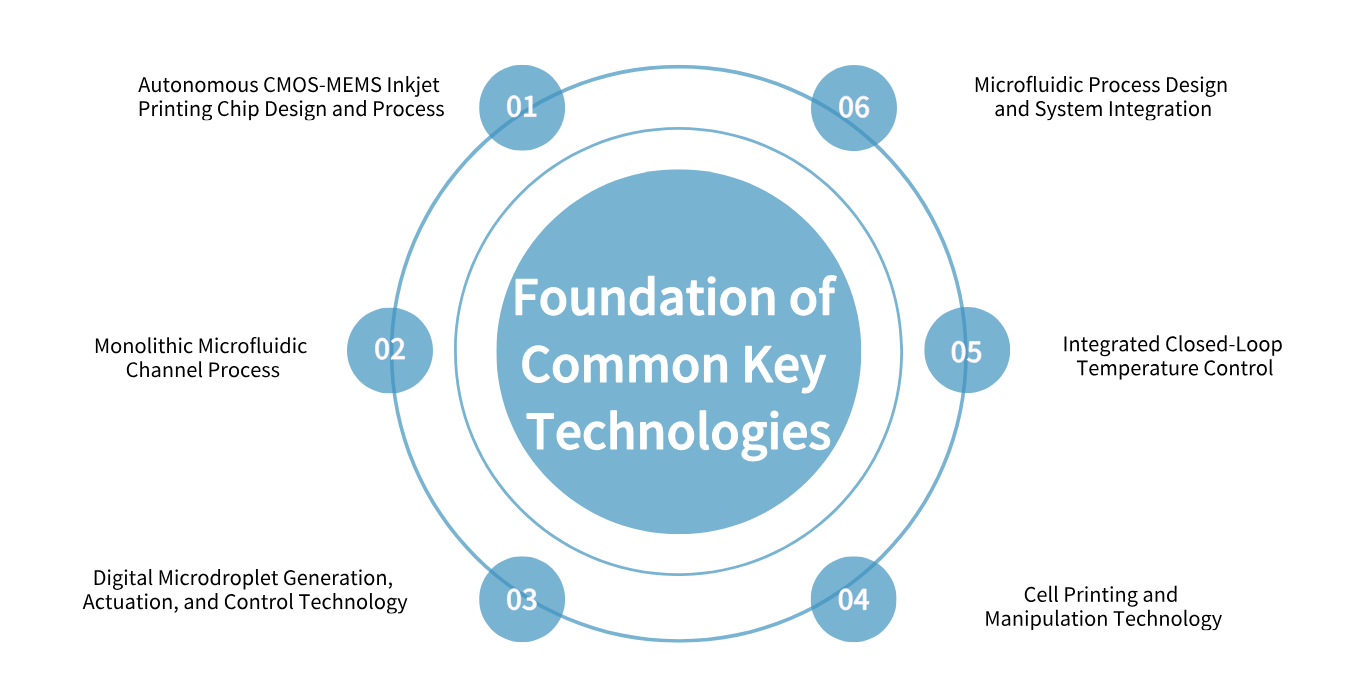
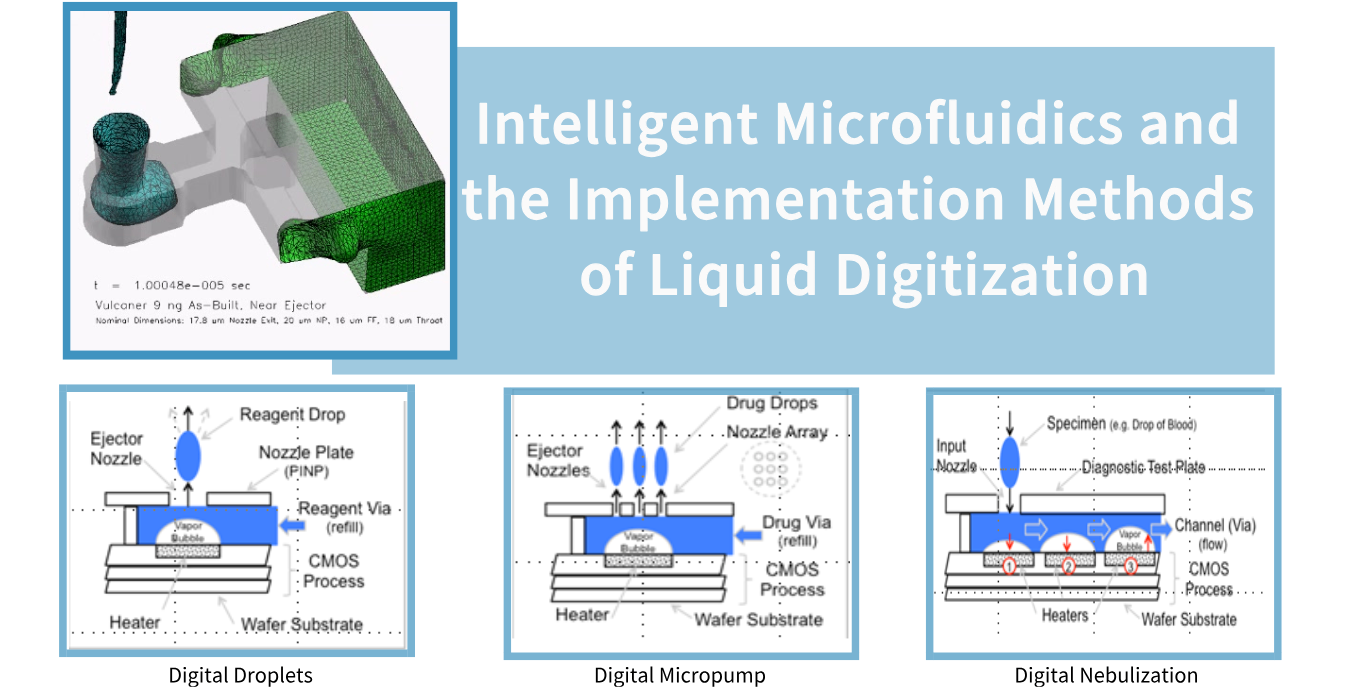
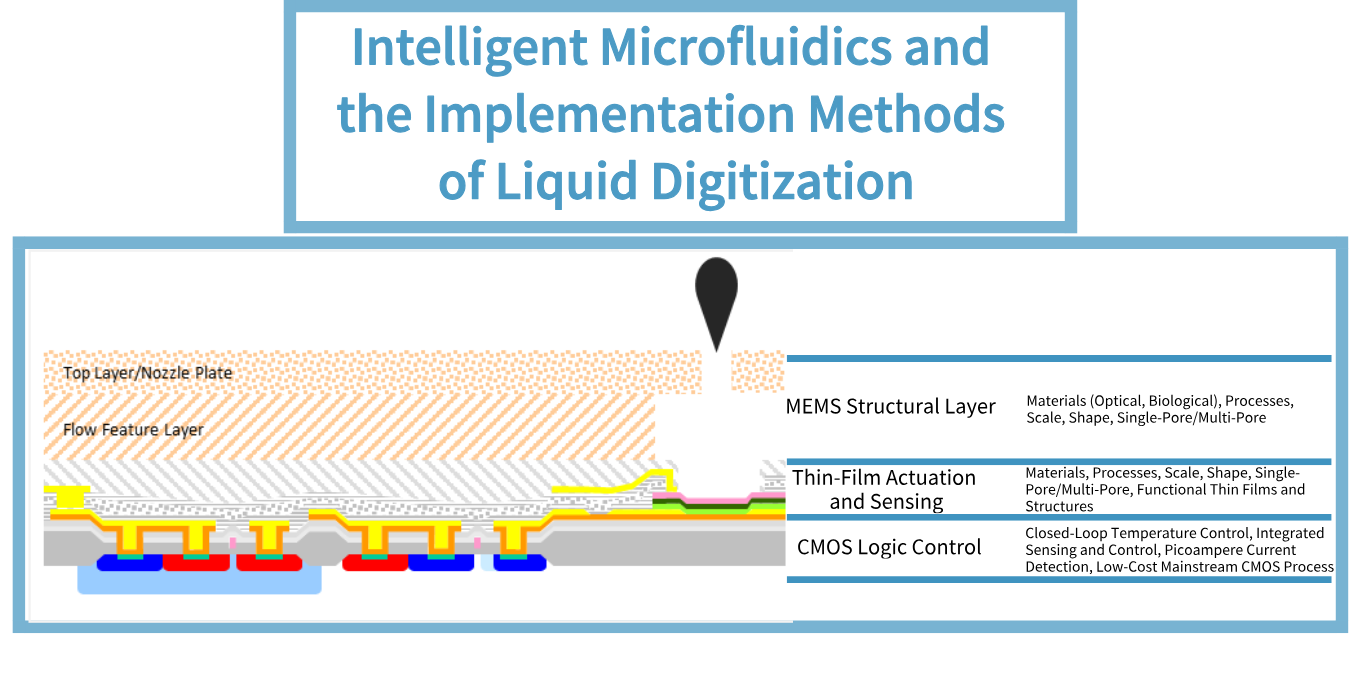
This is a team with outstanding professional capabilities, rich experience, and full of innovative vitality.
The team is composed of many outstanding professionals who have demonstrated profound professional knowledge and rich practical experience in strategic fields such as integrated circuits and bio-medicine.
Team members include senior individuals who have held key positions in internationally renowned companies, such as the head of Intel's Data Division in China and the General Manager of Data I/O Corporation in China; at the same time, there are industry experts with extensive resources and management experience in the investment field of integrated circuit industries; and technical backbones who have been engaged in research work in the field of bio-medicine for a long time, they play a core role in national special technology projects, or have published many papers with important academic value in international academic journals, or are committed to combining intelligent microfluidic technology with bio-medical applications. In addition, the team also has high-tech experts with profound professional knowledge in the fields of industry and healthcare, who are good at advanced research and development, product engineering development, and medical product registration.
